About The Recall
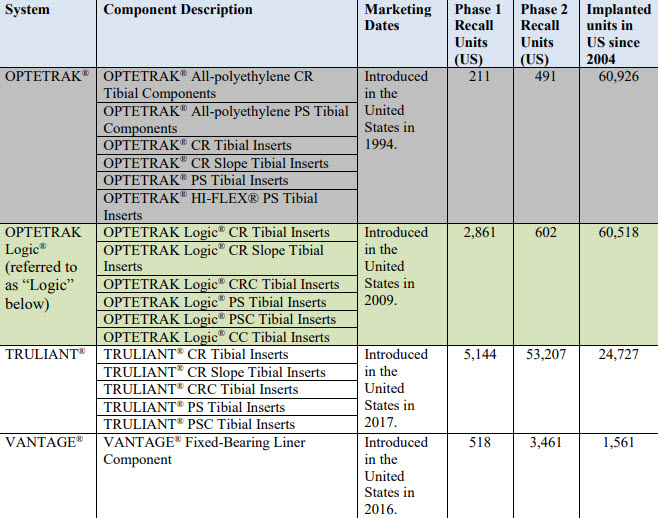
Recalled Devices
WCBJ News Report
Letter to Patients
Important patient notice regarding Exactech knee replacement devices
February 7, 2022
Dear valued patient,
Because the safety and health of our patients is our top priority, we are writing to inform you that between the years of 2004 and
2022, you received a specific type of total knee replacement that was manufactured by the orthopedic device company, Exactech,
Inc, headquartered in Gainesville, Florida, USA.
Exactech, Inc. has recently implemented a recall of one component and insert of the knee replacement device that you received and
is communicating with surgeons and patients who have utilized this knee replacement model.
Explanation of the recall:
As shown in the diagram below, a standard knee replacement has four parts:
- The femoral component (this is the metal piece that attaches to your thigh bone, also known as your “femur”)
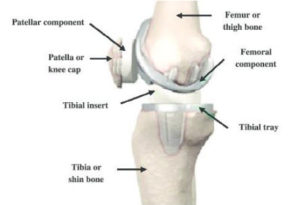
- The tibial tray (this is the metal piece that fits into your shin bone, also known as your “tibia”)
- The patellar component (this is the piece of plastic that fits onto your kneecap, also known as the patella)
- The tibial polyethylene (plastic) insert (this is the plastic that fits between the femoral component and tibial component and acts as the new cushion or cartilage for your replaced knee joint)
During a recent review of its knee implant manufacturing process, Exactech learned that one of the packaging layers for the plastic insert has been out of specification and may allow oxygen from the air to diffuse into the plastic insert prior to it being implanted in your knee. If a large amount of oxygen diffuses into the plastic insert while it’s being stored and before it is implanted, this can lead to a process called oxidation, which can cause the plastic to wear out earlier than expected or to become damaged after it is implanted into the patient’s body.
Exactech has found that the tibial plastic insert in the out of specification bag can wear out earlier than expected in some patients. Premature wear of the plastic insert of your knee replacement can lead to the need for additional surgery (also known as revision surgery). In those cases where the plastic has worn out earlier than expected or has been damaged, we will evaluate your knee replacement and decide whether additional treatment is needed. Determination of whether the plastic is worn is accomplished by examining your knee in the office and obtaining x-rays. After this evaluation is complete, we will decide if additional treatment, including revision surgery, is necessary.
See If You're Eligible for Compensation
See If You Have a Case
If you are experiencing pain, discomfort or other side effects, contact Farrar & Ball for more information.
Disclaimer
The information you obtain at this site is not, nor is it intended to be, legal advice. You should consult an attorney for advice regarding your individual situation. We invite you to contact us and welcome your calls, letters and electronic mail. Contacting us does not create an attorney-client relationship. Please do not send any confidential information to us until such time as an attorney-client relationship has been established.
Farrar & Ball, L.L.P. | Houston, TX | Ocala, FL